Some of our clients have questioned why so many separate bottles are needed for similar analyses, when it would be so much easier for their sample collection team to collect one bottle to be split by the lab upon receipt. For example, if a client wants to measure total and dissolved total mercury (THg) and methylmercury (MeHg), we would send out four fluoropolymer or glass bottles, one for each analysis. Is this overkill? Well, according the conclusions drawn from a recent paper co-authored by BAL’s VP of Operations, Annie Carter, it is not.
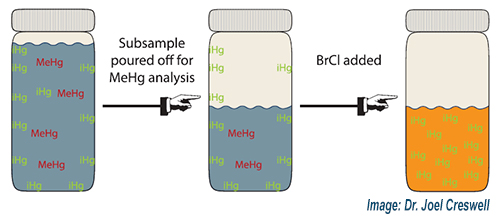
The lead author on this paper, titled Assessing bias in total mercury results after removing a subsample from the bottle, is former Brooks Rand Labs employee, Dr. Joel Creswell, who now works at the EPA. The research presented in the paper demonstrates that removing a subsample from the original bottle prior to addition of bromine monochloride (BrCl) can result in a positive bias for the THg concentration measured from the original container. The proposed mechanism for the bias is that ‘excess’ inorganic Hg adsorbs to the bottle walls, is not accounted for in the subsampled aliquot, and is then drawn into solution when BrCl is added. Collecting THg and MeHg samples in separate bottles whenever possible is recommended by the study authors. This same bias mechanism would apply if a subsample was removed from the original bottle to be filtered for dissolved Hg; therefore, collecting total and dissolved THg samples in separate bottles whenever possible is always advised by BAL scientists. Your BAL technical and project management team is ready to provide you with critical information like this prior to your next sampling event. Contact us today!
