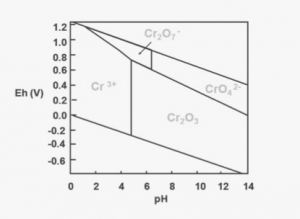
Chromium (Cr) is a polyvalent element and can exist in several distinct oxidation states, but only trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)] occur with any frequency in the natural environment. The mobility, bioavailability, and toxicity of chromium largely depend on which of these two chemical species is prevalent. The speciation of chromium is determined by the processes that control reduction/oxidation reactions in the environment. As can be seen by the adjacent simplified Pourbaix diagram of chromium species in water, species conversion can occur with only fairly minor changes in pH and Eh.
Chromium is a naturally occurring metal found in small quantities associated with other metals, particularly iron. It is commonly used for making steel and other alloys, bricks in furnaces, dyes and pigments, chrome plating, leather tanning, and wood preserving. Due to its extensive use in industrial processes, large quantities of chromium compounds are discharged into the environment.
For special applications that can benefit from the analysis of both Cr(III) and Cr(VI), Brooks Applied Labs offers a custom method to determine and quantify the concentrations of both species in aqueous samples. Our method couples ion chromatography (IC) with inductively coupled plasma – mass spectrometry (ICP-MS), and employs optimized interference removal technologies (DRC, CRC, and QQQ) parameters. This IC-ICP- MS configuration allows for multi-isotopic detection, high sensitivity, a wide linear dynamic range, and minimal polyatomic interferences. This allows us to separate and quantify chromium species in even the most complex matrices while maintaining ultra-low detection limits.
Brooks Applied Labs offers a proprietary method for chromium speciation analysis that can meet nearly any project quality objective, especially for projects that require ultra-low detection limits and involve samples more complex than drinking water. Accurate hexavalent chromium testing to determine ng/L levels is a major challenge because the existing methods have issues with selectivity, sensitivity, or biases. Both EPA Methods 7196A and 7199 rely upon a chemical reaction between diphenyl carbazide and spectrophotometric detection. Positive biases can be attributed with iron, molybdenum, manganese, vanadium, suspended solids, humic compounds, and other compounds that absorb light. Negative biases can also be encountered with elevated concentrations of transition metals, halogenated compounds, and samples with high buffering capacities. Although EPA Method 7199 uses chromatography to separate chemical and spectral interferences, complex sample matrices still require excessive dilutions which increases detection limits. Due to the inherent limitations with the aforementioned methods Brooks Applied Labs uses our own proprietary method using ion chromatography inductively coupled plasma mass spectrometry (IC-ICP-MS). Our clients rely upon our expertise and scientific understanding to provide the most representative analytical results possible and we will continue to offer only those methods which we know will meet our client’s data objectives.
To learn more about our innovative analytical methods and how they can benefit your projects,
contact us today.
 Chromium (Cr) is a polyvalent element and can exist in several distinct oxidation states, but only trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)] occur with any frequency in the natural environment. The mobility, bioavailability, and toxicity of chromium largely depend on which of these two chemical species is prevalent. The speciation of chromium is determined by the processes that control reduction/oxidation reactions in the environment. As can be seen by the adjacent simplified Pourbaix diagram of chromium species in water, species conversion can occur with only fairly minor changes in pH and Eh.
Chromium is a naturally occurring metal found in small quantities associated with other metals, particularly iron. It is commonly used for making steel and other alloys, bricks in furnaces, dyes and pigments, chrome plating, leather tanning, and wood preserving. Due to its extensive use in industrial processes, large quantities of chromium compounds are discharged into the environment.
For special applications that can benefit from the analysis of both Cr(III) and Cr(VI), Brooks Applied Labs offers a custom method to determine and quantify the concentrations of both species in aqueous samples. Our method couples ion chromatography (IC) with inductively coupled plasma – mass spectrometry (ICP-MS), and employs optimized interference removal technologies (DRC, CRC, and QQQ) parameters. This IC-ICP- MS configuration allows for multi-isotopic detection, high sensitivity, a wide linear dynamic range, and minimal polyatomic interferences. This allows us to separate and quantify chromium species in even the most complex matrices while maintaining ultra-low detection limits.
Brooks Applied Labs offers a proprietary method for chromium speciation analysis that can meet nearly any project quality objective, especially for projects that require ultra-low detection limits and involve samples more complex than drinking water. Accurate hexavalent chromium testing to determine ng/L levels is a major challenge because the existing methods have issues with selectivity, sensitivity, or biases. Both EPA Methods 7196A and 7199 rely upon a chemical reaction between diphenyl carbazide and spectrophotometric detection. Positive biases can be attributed with iron, molybdenum, manganese, vanadium, suspended solids, humic compounds, and other compounds that absorb light. Negative biases can also be encountered with elevated concentrations of transition metals, halogenated compounds, and samples with high buffering capacities. Although EPA Method 7199 uses chromatography to separate chemical and spectral interferences, complex sample matrices still require excessive dilutions which increases detection limits. Due to the inherent limitations with the aforementioned methods Brooks Applied Labs uses our own proprietary method using ion chromatography inductively coupled plasma mass spectrometry (IC-ICP-MS). Our clients rely upon our expertise and scientific understanding to provide the most representative analytical results possible and we will continue to offer only those methods which we know will meet our client’s data objectives.
To learn more about our innovative analytical methods and how they can benefit your projects, contact us today.
Chromium (Cr) is a polyvalent element and can exist in several distinct oxidation states, but only trivalent chromium [Cr(III)] and hexavalent chromium [Cr(VI)] occur with any frequency in the natural environment. The mobility, bioavailability, and toxicity of chromium largely depend on which of these two chemical species is prevalent. The speciation of chromium is determined by the processes that control reduction/oxidation reactions in the environment. As can be seen by the adjacent simplified Pourbaix diagram of chromium species in water, species conversion can occur with only fairly minor changes in pH and Eh.
Chromium is a naturally occurring metal found in small quantities associated with other metals, particularly iron. It is commonly used for making steel and other alloys, bricks in furnaces, dyes and pigments, chrome plating, leather tanning, and wood preserving. Due to its extensive use in industrial processes, large quantities of chromium compounds are discharged into the environment.
For special applications that can benefit from the analysis of both Cr(III) and Cr(VI), Brooks Applied Labs offers a custom method to determine and quantify the concentrations of both species in aqueous samples. Our method couples ion chromatography (IC) with inductively coupled plasma – mass spectrometry (ICP-MS), and employs optimized interference removal technologies (DRC, CRC, and QQQ) parameters. This IC-ICP- MS configuration allows for multi-isotopic detection, high sensitivity, a wide linear dynamic range, and minimal polyatomic interferences. This allows us to separate and quantify chromium species in even the most complex matrices while maintaining ultra-low detection limits.
Brooks Applied Labs offers a proprietary method for chromium speciation analysis that can meet nearly any project quality objective, especially for projects that require ultra-low detection limits and involve samples more complex than drinking water. Accurate hexavalent chromium testing to determine ng/L levels is a major challenge because the existing methods have issues with selectivity, sensitivity, or biases. Both EPA Methods 7196A and 7199 rely upon a chemical reaction between diphenyl carbazide and spectrophotometric detection. Positive biases can be attributed with iron, molybdenum, manganese, vanadium, suspended solids, humic compounds, and other compounds that absorb light. Negative biases can also be encountered with elevated concentrations of transition metals, halogenated compounds, and samples with high buffering capacities. Although EPA Method 7199 uses chromatography to separate chemical and spectral interferences, complex sample matrices still require excessive dilutions which increases detection limits. Due to the inherent limitations with the aforementioned methods Brooks Applied Labs uses our own proprietary method using ion chromatography inductively coupled plasma mass spectrometry (IC-ICP-MS). Our clients rely upon our expertise and scientific understanding to provide the most representative analytical results possible and we will continue to offer only those methods which we know will meet our client’s data objectives.
To learn more about our innovative analytical methods and how they can benefit your projects, contact us today.