No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
 BAL’s Jamie Fox will be speaking at this year’s DoD Environmental Monitoring & Data Quality (EMDQ) Workshop, which will take place May 13 – 16 in Orlando. Jamie will present “An Overview of Sequential Extraction Methods to assess Bioavailability and Mobility of Metals in Sediments.” This workshop is organized by the DoD Environmental Data Quality Workgroup and is for all interested members of the environmental community involved with DoD sites or projects including representatives from the DoD services. Please contact us if you will be attending or if you would like to receive a copy of the presentation after the conference.
BAL’s Jamie Fox will be speaking at this year’s DoD Environmental Monitoring & Data Quality (EMDQ) Workshop, which will take place May 13 – 16 in Orlando. Jamie will present “An Overview of Sequential Extraction Methods to assess Bioavailability and Mobility of Metals in Sediments.” This workshop is organized by the DoD Environmental Data Quality Workgroup and is for all interested members of the environmental community involved with DoD sites or projects including representatives from the DoD services. Please contact us if you will be attending or if you would like to receive a copy of the presentation after the conference.
 Any time metals are an environmental contaminant of concern, the predictable approach is to look at the total metals concentrations and determine a path forward. Whether it be determining risk due to toxicity, selecting a remediation plan, or establishing a regulatory permit limit, the decisions that are made assuming that all molecular forms of a metal have the same physical and chemical properties can have a number of dire consequences. Elemental speciation should be the first choice to avoid such erroneous and potentially costly assumptions from being made. There are many methods for the speciation of metal in solid matrices, but often times the most informative and cost-effective approach is to use selective sequential extractions.
Any time metals are an environmental contaminant of concern, the predictable approach is to look at the total metals concentrations and determine a path forward. Whether it be determining risk due to toxicity, selecting a remediation plan, or establishing a regulatory permit limit, the decisions that are made assuming that all molecular forms of a metal have the same physical and chemical properties can have a number of dire consequences. Elemental speciation should be the first choice to avoid such erroneous and potentially costly assumptions from being made. There are many methods for the speciation of metal in solid matrices, but often times the most informative and cost-effective approach is to use selective sequential extractions.
Selective sequential extractions (SSE) have a number of useful applications for nearly any industry trying to understand the nature of the metals that are present in environmental samples and to predict or model how they might move through the environment (mobility), be metabolized by surrounding organisms (bioavailability), and bioaccumulate or biomagnify up the food chain.
The basic premise of SSE is that a representative solid sample (soil, sediment, tissue, etc.) undergoes an initial extraction which is typically representative of what might be easily transported or dissociated from the solid matrix. This is the “mobile” fraction. Depending on the element, there may be species-specific information that can be elucidated from this fraction, as well as from subsequent fractions. Next, this same sample aliquot is extracted with a second solution that generally represents something that is more strongly bound than the first fraction but still relatively available to the surrounding environment depending on site conditions. Each subsequent extraction uses a “stronger” reagent solution, leading to the final extraction which is generally the residual fraction and represents strongly-bound and generally immobile metal complexes in the solid phase. It is very important to note is that if subsurface samples or any other type of sample that is in relatively anoxic or from reducing conditions are collected, the use of anoxic sample collection techniques and notification of the laboratory that glove boxes must be used to maintain anoxic conditions may be required. Otherwise, sample results will not be representative of site conditions.
There are limitations of SSE that are important for anyone requesting these methods to be aware of. The first is that “one size does not fit all.” The SSE methods that exist in the literature and in published methods are intended for a very specific element or set of elements. When any of these SSE methods are applied to elements they were not intended for or have not been studied in detail, decisions based on this data may be questionable. BAL can provide guidance with the selection of the most appropriate method(s). The second is that these are operationally-defined methods, which means that the results for each fraction are defined as what was extractable by that reagent solution for that particular solid sample. This can be different for each set of samples. The information that is present in the literature or method regarding what types of species are expected to be extracted in each fraction can vary somewhat, so that a particular species may be present in a previous sequential extraction or may come out in a later extraction. The strength of these methods is to provide a strong indication of which metals are highly mobile, which are bioavailable, and which are not. Brooks Applied Labs currently has selective sequential extraction procedures for the fractionation of Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Hg, Pb, Mo, Cd, and Tl.
 Brooks Applied Labs was recently mentioned in a Canadian Broadcasting Company news segment on their investigation into arsenic concentration of baby foods sold in Canada. BAL performed the testing which showed some baby foods contained inorganic arsenic above the US FDA guideline and the EU regulatory limit of 100 ppb. While minimizing exposure to this harmful element is beneficial to people of all ages, it is especially important for infants due to their smaller size and proportionally larger calorie requirements. Our testing showed some baby foods contained inorganic arsenic above the US FDA guideline and the EU regulatory limit of 100 ppb. Read more about the CBC Marketplace investigation here, or watch the full news program here.
Brooks Applied Labs was recently mentioned in a Canadian Broadcasting Company news segment on their investigation into arsenic concentration of baby foods sold in Canada. BAL performed the testing which showed some baby foods contained inorganic arsenic above the US FDA guideline and the EU regulatory limit of 100 ppb. While minimizing exposure to this harmful element is beneficial to people of all ages, it is especially important for infants due to their smaller size and proportionally larger calorie requirements. Our testing showed some baby foods contained inorganic arsenic above the US FDA guideline and the EU regulatory limit of 100 ppb. Read more about the CBC Marketplace investigation here, or watch the full news program here.
Compared to other grains, rice is known to contain higher levels of the toxic and carcinogenic element arsenic. But the arsenic concentrations and the proportion of the more harmful inorganic arsenic forms can vary widely in rice depending on a number of factors, including where the rice is grown, the growing conditions, and how it is processed after harvesting. Quantifying the more toxic inorganic arsenic forms via speciation analysis, which Brooks Applied Labs has been performing for decades, is therefore necessary to assess arsenic-related risks due to rice consumption.
 Team Brooks Applied Labs has been incredibly busy the past several weeks, with conference presentations locally, nationally, and internationally!
Team Brooks Applied Labs has been incredibly busy the past several weeks, with conference presentations locally, nationally, and internationally!
Our scientists and technical specialists strive to develop new methods and provide educational opportunities to meet the needs of our current and future clients! Contact us to learn more about any of our services.

Selenium testing for total recoverable concentrations by conventional ICP-MS techniques is extremely prone to mass spectral interferences. The plasma gas (argon) and constituents of the sample matrix (calcium, carbon, chloride, sulfur, etc.) can easily combine to form polyatomic ions with the same mass-to-charge ratios as the various isotopes of selenium, resulting in false-positives and elevated detection limits.
The table below lists the different isotopes of selenium and the common interferences that can affect measurements at those isotopes.
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.

Once released into the environment, lead is persistent and can accumulate in soil, water, and living organisms. It is particularly concerning because of its neurotoxicity, especially in children, where exposure can result in cognitive impairments, developmental delays, and other health issues. In adults, lead exposure can contribute to cardiovascular problems, kidney damage, and reproductive toxicity.
The accurate measurement of lead levels is essential for assessing exposure risks, complying with regulatory standards, and guiding remediation efforts. At Brooks Applied Labs, we specialize in ultra-low-level lead analysis and lead speciation in a wide range of sample matrices. Our advanced analytical methods ensure precise and reliable results to help protect human health and the environment. Please contact us to learn more about lead testing options and how we can assist with your specific analytical needs.
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
In order to standardize the approach for compliance analyses to support the ICH Q3D Guideline for Elemental Impurities, the U.S. Pharmacopeia (USP) generated the guidance methods USP 232 and 233. The ICH guideline applies to new finished drug products (as defined in ICH Q6A and Q6B) along with new drug products containing existing drug substances. Brooks Applied Labs is a CRO specializing in elemental and metalloid speciation analyses, period. Our focus makes your decision regarding partnering on ICH Q3D compliance an easy one.
The guideline, not the associated USP monograph, stipulates exactly how to address spectral interferences associated with mass spectrometric measurements. Elements in the mid-range mass region (30 amu -114 amu) are more prone to positive biases than heavier elements using mass spectrometry. Brooks Applied Labs uses some of the most advanced analytical technologies, inductively coupled plasma triple quadrupole mass spectrometry (ICP-QQQ-MS), to deliver results of unparalleled quality. Research at Brooks Applied Labs has identified that operating ICP-MS instruments in KED mode, which most other laboratories are limited to, cannot properly remove all relevant spectral interferences. In addition, by using acid cleaned labware integrated into a robust quality system, biases are documented and mitigated on a continual basis reducing your risk when selecting a CRO.
Brooks Applied Labs has been audited by our pharmaceutical clients, third party accreditation services, as well as the FDA, which play an invaluable role in our desire to continue to improve our quality systems and support our clients’ needs.
| Classification | Elemental Impurities |
| Class 1 | As, Cd, Hg, and Pb |
| Class 2A | Co, Ni, and V |
| Class 2B | Ag, Au, Ir, Os, Pd, Pt, Rh, Ru, Se, and Tl |
| Class 3 | Ba, Cr, Cu, Li, Mo, Sb, and Sn |
| Other Elements | |
| Al, B, Ca, Fe, K, Mg, Mn, Na, W, and Zn | |
The ICH Q3D guideline should be consulted with regards to performing a risk assessment for the listed elemental impurities. Tables presenting the permitted daily exposures and extrapolated permitted concentrations are listed in the tables below. The tables are for reference purposes only and reflect the ICH Q3D Guideline as of January 1, 2019.
|
Permitted Daily Exposures for Elemental Impurities1 |
||||
| Element | Class | Oral PDE (μg/day) | Parenteral PDE (μg/day) | Inhalation PDE (μg/day) |
| Cd | 1 | 5 | 2 | 2 |
| Pb | 1 | 5 | 5 | 5 |
| As | 1 | 15 | 15 | 2 |
| Hg | 1 | 30 | 3 | 1 |
| Co | 2A | 50 | 5 | 3 |
| V | 2A | 100 | 10 | 1 |
| Ni | 2A | 200 | 20 | 5 |
| Tl | 2B | 8 | 8 | 8 |
| Au | 2B | 100 | 100 | 1 |
| Pd | 2B | 100 | 10 | 1 |
| Ir | 2B | 100 | 10 | 1 |
| Os | 2B | 100 | 10 | 1 |
| Rh | 2B | 100 | 10 | 1 |
| Ru | 2B | 100 | 10 | 1 |
| Se | 2B | 150 | 80 | 130 |
| Ag | 2B | 150 | 10 | 7 |
| Pt | 2B | 100 | 10 | 1 |
| Ll | 3 | 550 | 250 | 25 |
| Sb | 3 | 1200 | 90 | 20 |
| Ba | 3 | 1400 | 700 | 300 |
| Mo | 3 | 3000 | 1500 | 10 |
| Cu | 3 | 3000 | 300 | 30 |
| Sn | 3 | 6000 | 600 | 60 |
| Cr | 3 | 11000 | 1100 | 3 |
|
1Values in this table have beeb taken from Table A.2.1 from ICH Q3D. Please see the respective ICH guidelines for additional information. |
||||
|
Permitted Concentrations of Elemental Impurities2 |
||||
| Element | Class | Oral PDE (μg/day) | Parenteral PDE (μg/day) | Inhalation PDE (μg/day) |
| Cd | 1 | 0.5 | 0.2 | 0.2 |
| Pb | 1 | 0.5 | 0.5 | 0.5 |
| As | 1 | 1.5 | 1.5 | 0.2 |
| Hg | 1 | 3 | 0.3 | 0.1 |
| Co | 2A | 5 | 0.5 | 0.3 |
| V | 2A | 10 | 1 | 0.1 |
| Ni | 2A | 20 | 2 | 0.5 |
| Tl | 2B | 0.8 | 0.8 | 0.8 |
| Au | 2B | 10 | 10 | 0.1 |
| Pd | 2B | 10 | 1 | 0.1 |
| Ir | 2B | 10 | 1 | 0.1 |
| Os | 2B | 10 | 1 | 0.1 |
| Rh | 2B | 10 | 1 | 0.1 |
| Ru | 2B | 10 | 1 | 0.1 |
| Se | 2B | 15 | 8 | 13 |
| Ag | 2B | 15 | 1 | 0.7 |
| Pt | 2B | 10 | 1 | 0.1 |
| Ll | 3 | 55 | 25 | 2.5 |
| Sb | 3 | 120 | 9 | 2 |
| Ba | 3 | 140 | 70 | 30 |
| Mo | 3 | 300 | 150 | 1 |
| Cu | 3 | 300 | 30 | 3 |
| Sn | 3 | 600 | 60 | 6 |
| Cr | 3 | 1100 | 110 | 0.3 |
|
2Values in this table have been taken from Table A.2.2 from ICH Q3D. Please see the respective ICH guideline for additional information. |
||||
Brooks Applied Labs has been audited by our pharmaceutical clients, third party accreditation services, as well as the FDA, which play an invaluable role in our desire to continue to improve our quality systems and support our clients’ needs. Our approach to quality is dynamic with the understanding that the regulatory environment can, and will, change necessitating a proactive approach by both our clients and Brooks Applied Labs.
Contact us to find out how your quality and process objectives can be met more efficiently than ever.
The nature of biopharmaceuticals requires growth media to support the functionality of the cell growth and production of the desired biological expression. As with higher trophic organisms, micronutrients are necessary for optimal growth and viability of cells, whether the biopharmaceutical production uses animal or plant-based cell lines. This includes mAb, CAR-T, viral vector, and future pharmaceutical drug opportunities. Each one having its own special demands and concerns.
Improper micronutrient controls in biopharmaceutical production can result in out of specifications (OOSs), lower yield, decreased profitability, and worst of all, interruptions in availability of life saving drugs for patients. Thus, it is of paramount importance for the biopharmaceutical industry, as a whole, to implement appropriate quality practices associated with their delivery and control.
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
Micronutrients are dietary components, often referred to as vitamins and minerals, which although only required by organisms in small amounts, are vital to development, activity, and viability. Minute changes to micronutrient levels during all phases of biopharmaceutical investigations and production stages can have significant impacts driving OOS and R&D concerns. The quantitation of micronutrients, specifically minerals and metal bearing vitamins, as well contaminants that are known to be relevant to all aspects of the biopharmaceutical industry is what Brooks Applied Labs specializes in.
The quantitation of micronutrients, specifically minerals and metal bearing vitamins, as well contaminants that are known to be relevant to all aspects of the biopharmaceutical industry, is what Brooks Applied Labs specializes in.
Micronutrients are a small component of chemically defined media but play a key role in optimization and control of bioreactors. Unfortunately, due to the relatively low concentrations of certain minerals in the chemically defined media, the contribution from all sources of the process must be taken into consideration. Thus, a holistic approach to micronutrients is necessary for controlling the process, whether the project is in the R&D phase, FDA filing, or final CGMP production phase.


Many of our methods are validated in accordance with the International Conference of Harmonization (ICH) and Code of Federal Regulations (CFR). This not only lends confidence in our quality systems but also ensures our clients can apply analytical results in a standardized basis throughout their processes.
Brooks Applied Labs has been audited by our pharmaceutical clients, third party accreditation services, as well as the FDA, which play an invaluable role in our desire to continue to improve our quality systems and support our clients’ needs.
Brooks Applied Labs has been audited by our pharmaceutical clients, third party accreditation services, as well as the FDA, which play an invaluable role in our desire to continue to improve our quality systems and support our clients’ needs. Our approach to quality is dynamic with the understanding that the regulatory environment can, and will, change necessitating a proactive approach by both our clients and Brooks Applied Labs.
Contact us to find out how your quality and process objectives can be met more efficiently than ever or read more about our services by clicking on any of our brochures below.
Elemental analyses of small molecule pharmaceuticals pose special challenges in accordance with the business cycle of the investigation. Investigations associated with final drug products tend to be more straightforward than those involved with R&D due to the reduction in variables. Regardless of the project stage, scientists at Brooks Applied Labs have experience with your concerns allowing us to produce high quality analytical results the industry relies upon.
Catalysts used for chemical syntheses have different chemical properties than most of the transition metals necessitating a greater focus on science. Furthermore, the chemical and thermal demands for cleaving hydrocarbon bonds while stabilizing target elements must be taken into consideration for normalizing the hydrocarbon input into analytical systems using inductively coupled plasma technologies as well as mitigating analyte loss in analytical components.

My synthesis reaction only generates milligrams of material before the blow-down procedure. How can I achieve quantitative results with such a low mass?
The concept of limited mass often confounds research scientists when performing viability tests on resins for catalyst recovery. By using the appropriate sampling vessels and experimental techniques the issue of mass limitations can typically be overcome. Each experiment has its own set of variables and limitations which is why collaborating with Brooks Applied Labs, before project inception, is important. In certain instances, mass limitations cannot be overcome limiting a comparative evaluation to an absolute mass basis for target analytes.
At Brooks Applied Labs we understand that time is of the essence. At the onset of each project our specialists discuss your data objectives and expectations to ensure your projects are delivered to your satisfaction.
The reactivity of certain resins when exposed to strong acids and heat must be understood to safely handle and prepare materials prior to analyses. The safety of our personnel is of the utmost importance when supporting capacitance investigations for resin materials. Through our experience and knowledge, scientists at Brooks Applied Labs have developed scientific approaches to both maximize our safety as well as to produce analytical results to meet your data objectives.

Halogens (Cl, Br, I, S, and P) can play a key role in your processes, whether they are present in your raw materials, intermediates, active pharmaceutical ingredient (API), or final drug product. Brooks Applied Labs has supported both elemental quantitation of halogens as well as molecular analyses using advanced techniques such as ion chromatography in-line ultra violet/visible spectrophotometry inductively coupled plasma dynamic reaction cell mass spectrometry (IC-UV/Vis-ICP-DRC-MS). This is another example of how our scientific acumen is put to the test to meet our client’s data objectives.
Whether your project involves investigation of elemental impurities or is driven by development and processes, contact Brooks Applied Labs today to experience the difference in CROs.
Quality aspects of micronutrients’ role in biopharmaceutical production demands a holistic approach to the supply chain. Minute differences in concentrations of certain minerals in the low parts per billion (µg/L) range can have sweeping effects including increasing titer, meeting specifications, and out of specifications (OOSs). Unfortunately, micronutrients can be present in nearly all aspects of production as contaminants. Thus, a new approach to quality controls and risk assessments are necessary to ensure the viability of processes.
Minute differences in concentrations of certain minerals in the low parts per billion (µg/L) range can have sweeping effects including increasing titer, meeting specifications, and out of specifications (OOSs).
The International Conference of Harmonization (ICH) and Code of Federal Regulations (CFR) provide guidance regarding quality aspects of pharmaceutical drug manufacturing and management. Identification of variables and performing appropriate risk assessments, however, is the responsibility of drug companies. An important aspect of this process, which is often overlooked, is properly identifying data objectives and selecting a CRO that can meet and exceed current and future analytical demands. Brooks Applied Labs uses the some of the most advanced analytical techniques to support the quantitation of elemental micronutrients and contaminants using inductively coupled plasma triple quadrupole mass spectrometry (ICP-QQQ-MS). We are experts in the field of elemental analyses, so you do not have to be. However, if you would like to learn more click here.
Considering, not all pharmaceutical companies have the personnel with the knowledge and experience to not just understand the role of all micronutrients but also how to integrate those variables into their quality systems surfaces a need. Brooks applied Labs can assist with this.
The first stages of quality systems are to identify variables associated with the processes and perform a risk assessment. What variables contribute to micronutrient concentrations?
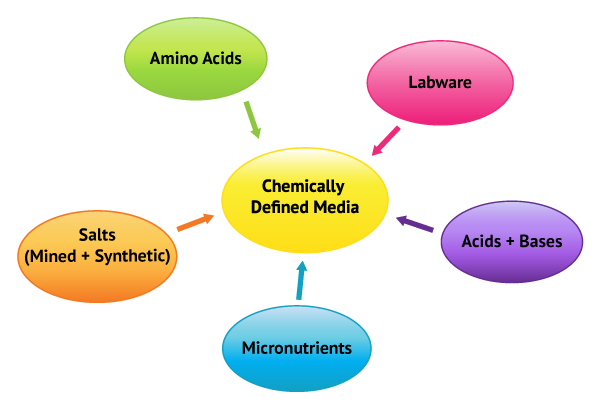
Along with chemicals that are used for chemically defined media all labware can also contribute to micronutrient loading. More often than not, the concept of labware is limited to pipettes, PET bottles, and stir rods. However, it should include all physical materials that come in contact with all powders, liquids, and cell lines. The list can become quite extensive but important, nonetheless. One excellent example of a labware issue BAL encountered was with single use bioreactors. The client continued to experience OOSs and BAL helped elucidate that Cu contamination from the disposable bag was the result. In another instance, a bioreactor manufacturer used high iron stainless steel resulting in oxidation and, as you can guess, continued OOSs.
One excellent example of a labware issue BAL encountered was with single use bioreactors. The client continued to experience OOSs and BAL helped elucidate that Cu contamination from the disposable bag was the result.
How much material should be collected and stored for possible future analyses as a retain? This brings us to the next variable to understand and that is homogeneity. Impurities in powdered chemicals are inherently heterogeneous up to a specific mass. This is dependent on how the powder was milled, the source of the chemical (synthesized or mined), and level of hydration. If the supplier cannot provide a mass limitation for homogeneity, a study must be performed to ascertain how much material is required to produce a representative subsample. Brooks Applied Labs can help you with this.

The concept of retains and homogeneity are especially important in the R&D phase for biopharmaceuticals. The variable of uncontrolled micronutrients should never be cause for concern whether a cell line failed to produce the anticipated molecular expression. After all, what company would want to miss out on the next billion-dollar blockbuster drug because a handful of samples were not analyzed?

Inductively coupled plasma – mass spectrometry (ICP-MS) is widely recognized as one of the most accurate and precise analytical techniques for the determination of many trace metals in a wide variety of sample types. However, analyses for a small number of these elements using a conventional instrument configuration have also been persistently challenging due to spectral interferences that can severely compromise the accuracy of reported results.
These spectral interferences occur due to the formation of polyatomic ions that have the same mass-to-charge ratio (m/z) as the analytes of interest. This causes erroneously large signals for the intended analyte at the detector and results in unnecessarily elevated detection limits and mistakenly elevated final concentrations.

Polyatomic ions form when the various constituents of the plasma gas, preparation reagents, and the sample matrix combine in the ion beam and prior to reaching the detector. Procedurally introduced elements, such as the argon that fuels the plasma, as well as the chlorine, hydrogen, nitrogen, and oxygen found in the diluents and acids used in sample preparation, unavoidably contribute to the formation of many of these polyatomic ions, even in relatively low-level samples.
The analyses of samples that contain abundant amounts of particular elements, such as calcium, carbon, chlorine, magnesium, potassium, sodium, or sulfur, can dramatically further increase the formation of polyatomic ions to levels that can easily exceed even those of the analytes of interest. Of course, these elements are present in almost all chemically defined media for cell cultures making it imperative to use the best technology available to mitigate analytical bias.
“Interferences and bias associated with ICP-MS technology is serious business. ICP-QQQ-MS technology allows BAL to itigate these interferences while achieving ng/L detection limits.
What sets the ICP-QQQ-MS analytical platform apart from other ICP-MS instruments is the HMI system and the quadrupole that precedes the CRC. This allows only specific masses to enter the cell, resulting in a more controlled and efficient interference removal in reaction mode. Most mass spectrometers only contain a quadrupole mass analyzer after the sample stream exits the CRC. Furthermore, the support of mass filtering prior to the CRC allows our scientists to scan the periodic table during the analysis of a specific element to ascertain what, if any, interferences are caused by the plasma effects or the interaction of the reaction gases with the ion beam.
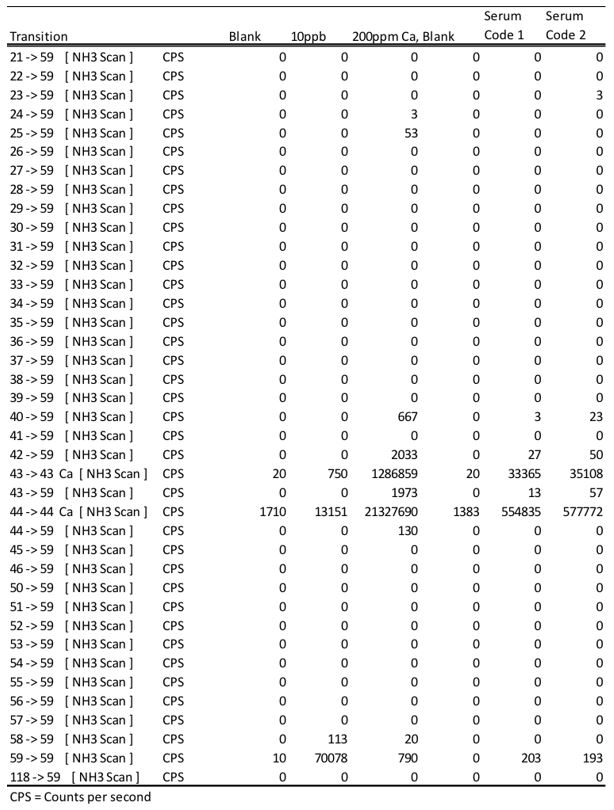
Below is an example of an investigation using the mass scanning capacity of the QQQ to elucidate the formation of nitrogen analogues which can interfere with cobalt quantitation. It was determined that calcium analogues were the primary contributor for a positive bias when operating the CRC with ammonia gas. This is another example how our scientists go above and beyond to understand the benefits and limitations of technologies before application to our clients.
From education, variable recognition, contaminant source identification, and analytical services to support all aspects of elemental analyses in your systems, Brooks Applied Labs is your solution provider. If you do not currently have a Master Service Agreement (MSA) with us feel free to contact us to get started.